- Academic Programs & Support
- Office of the Provost
- Research at WCU
- Sponsored Research
- Research Compliance
- Human Subjects Research
- Institutional Review Board FAQs
Institutional Review Board FAQs
Below are Frequently Asked Questions related to basic policies and procedures that govern the IRB review and approval process.
While we serve a regulatory function, we take a collegial approach to consultation with researchers and their staff. Please feel free to call on us as you navigate the process. We are happy to respond to your inquiries.
IRB Overview
IRB stands for "Institutional Review Board." The IRB is a committee established to review and approve applications for research projects involving human subjects, regardless of funding source. The IRB reviews all projects conducted at or under the auspices of WCU by WCU personnel. The primary purpose of the IRB is to protect the rights and welfare of human participants.
Research is defined as a "systematic investigation, including research development, testing
and evaluation, designed to develop or contribute to generalizable knowledge." Generalizable
knowledge is not defined solely as an intent to publish.
Human subjects are "living individuals about whom an investigator conducting research i) obtains
information or biospecimens through intervention or interaction with the individual
OR ii) obtains, uses, studies, analyzes or generates identifiable private information
or biospecimens."
Projects that meet the criteria of both definitions require review by the IRB. If
you are unsure whether your project involves research with human subjects please contact IRB@wcu.edu for help making this determination.
You may also use this Flowchart to help guide you.
The IRB must review and approve the project prior to investigators beginning any proposed research activity (including recruitment of subjects).
If students will only be presenting the results of their study in a classroom setting, then the project does not meet the definition of research because the projects are not contributing to generalizable knowledge. However, instructors should be aware that if IRB approval is not obtained prior to study initiation results or data may not be disseminated outside the classroom for any purpose, even if a project generates interesting results. Instructors and students should discuss and consider these limitations prior to beginning a project. If a classroom project does not require IRB review, it is the responsibility of the instructor to use good ethical judgment in determining whether a proposed student project is appropriate.
Quality improvement projects are not subject to IRB review and do not need to be submitted to the IRB. Use the following chart to determine whether your project is considered reseach. Please note that some quality improvement projects have an element of research. Any quality improvement project that includes research components should be submitted to the IRB for review. If you need assistance determing whether your project needs review, please contact IRB@wcu.edu
PI Eligibility and Role of Investigators
All submitted projects must list a PI. Any full-time WCU faculty or staff may serve as PI. Students may serve as co-PI, but the faculty advisor must be listed as the PI and must review all proposal documents prior to submission.
The PI is responsible for assuming meaningful supervision for other investigators
and each student researcher listed on the protocol. The PI must be reasonably available
to assist in the resolution of and reporting of any problems that may arise during
the course of the research.
The PI is responsible for imparting to students an understanding of the ethical principles
of human research to which the university adheres.
If a student is conducting research or a class activity that falls within the IRB's
purview, the faculty member teaching the course must ensure that the student completes
appropriate training and secures IRB approval before beginning the study or activity.
The PI also has primary responsibility for overseeing and monitoring the conduct of
research in its entirety and for ensuring that students adhere to the approved protocol
(e.g. data storage, retention, and disposal).
Collaborative Research
For unaffiliated investigators that have an institutional IRB: collaborating institutions
may have the option to enter into a Reliance Agreement, (or IRB Authorization Agreement),
which allows one IRB to provide review and oversight for the study. The IRB Office
will work with investigators to determine if single IRB review is feasible. If a WCU
investigator will rely upon the approval of an external IRB, the investigator should
provide all related study documentation (e.g. application form, consent form, advertisements)
approved by the partnering agency.
Further information about collaborative research can be found on the Collaborative Research website.
Individuals unaffiliated with WCU who will be listed as members of the study team must sign and submit an Individual Investigator Agreement in order to perform work on the project. This form does not apply if the external investigator has an institutional IRB. Individual Investigators must also completed WCU's CITI human subjects training requirement.
Unaffiliated investigators, who wish to conduct research that takes place on the WCU campus or that involves WCU faculty, students, or staff should submit a copy of the application and approval letter from their institution's IRB to the WCU IRB by email. Following review for compliance with WCU policy for researching involving humans, the IRB will grant permission to begin the research.
Review Process
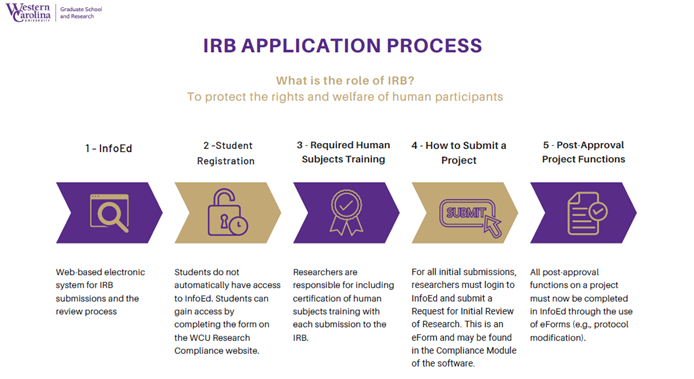
WCU uses an electronic system called InfoEd, to process and review all IRB applications. All project materials must be submitted through this system.
Projects will undergo one of the following types of review: exempt review, expedited review, or full-board review. Studies that meet one or more exempt criteria will be reviewed via the exempt review process. Studies that contain no more than minimal risk and meet expedited criteria will undergo review by one designated member of the IRB. Studies involving vulnerable or special populations, or involving more than minimal risk to the subjects will undergo review by the convened IRB.
Exempt and Expedited reviews generally take about 2-4 weeks. However, during University holidays and breaks the process may take longer. Projects requiring full-board review must be submitted two weeks prior to the next scheduled meeting and will be placed on the agenda once received.
The project is received by the IRB administrative office where your project will undergo an administrative review for completeness and clarity and the appropriate level of review will be determined. Studies requiring expedited review will be forwarded to a designated reviewer. Studies requiring full-board review will be assigned to a primary reviewer and will be distributed to the entire IRB committee. The application materials will be reviewed at a scheduled IRB meeting. Investigators will be notified via InfoEd if revisions are necessary or when the study has been approved.
You will receive notification of approval via InfoEd and email. Approval letters and approved study materials will be maintained in InfoEd, and are available for download by the investigator at any time.
Yes, the IRB may elect to disapprove a project. Typically the designated reviewer will work with the investigators to develop an acceptable protocol. However, if the investigator and the IRB are unable to establish a protocol that will be supported by the IRB, the Full-Board can vote to disapprove the project.
Informed Consent
Consent is a continuing process that starts before any forms are signed and continues until the individual's participation is complete. It involves meeting with a potential participant, determining whether s/he is capable of giving consent, discussing the purpose, risks, and benefits of participation, etc. Its goal is to assure that prospective participants can knowledgeably and voluntarily, without coercion, decide whether to participate. Consent is required for every participant unless the consent or one of its elements has been waived by the IRB. If a project is determined to be Exempt, a consent process is still required if direct interaction with human subjects will occur.
When the research participant is child (<18 years old), both permission from the parents using the informed consent process and informed assent from the child must be obtained, unless the IRB grants a waiver of consent. The assent process must be tailored to be age appropriate to the child. For example, if obtaining informed assent from a 7 year old, the assent form must be written at their reading level.
There are 8 required elements for informed consent, with other elements required dependent
on the project. A brief list of the required elements is below:
1. Statement the activity is research, the purpose of the research, the duration,
a description of procedures and identification of any experimental procedures
2. A description of any foreseeable risks or discomforts
3. A description of any foreseeable benefits to the individual or to society
4. Disclosure of any alternative treatments or procedures that might be advantageous
5. A statement describing the extent to which confidentiality will be maintained
6. For more than minimal risk studies, a description of any compensation or available
treatments in the event of an injury
7. Contact list for who to contact with questions about the research study or treatment
as a subject
8. A statement that participation is voluntary, that refusal to participate involves
no penalty or loss of benefits to which the subject is entitled, and the subject may
discontinue participation at any time with no penalty or loss of benefits
Confidentiality and Privacy
Anonymous data cannot be linked, even indirectly or through a code, back to a subject. Confidential data may be linked to a code or directly identifiable to the research, but the subject's privacy is protected and the identity is not disclosed outside the research team.
Project Lifecycle
The approval period for projects undergoing full-board review is for no more than a year. Project renewal must be submitted prior to the expiration date. After the initial approval, an Application for Continuing Review may be submitted for years 2 and 3 of the research. To begin the 4th year of research, you must submit an Application for initial review as part of de novo review.
After a research study has been reviewed and approved by the IRB, the investigator MUST conduct the study exactly as approved by the IRB. No changes in approved research should be initiated without prior IRB review and approval, except where necessary to eliminate apparent immediate hazards to participants (these cases must be reported to the IRB immediately). The Request for Modification form is used to request any changes to your research protocol including changes to approved documents such as consent forms, recruitment materials, or instruments to be used in the study. Changes may not be implemented until approval is received from your IRB reviewer.
Researchers must promptly report to the IRB any serious or continuing non-compliance with federal regulations or university policies, any injuries to subjects, or unanticipated problems.
Researchers must submit a Report of Protocol Deviation or Violation within 10 business
days of a protocol deviation or violation:
• A Protocol Deviation is an inadvertent act (from the perspective of the PI and study
staff) in which the protocol is not followed. Examples that are reportable to the
IRB include the accidental loss of consent forms, or study materials with participant
information, or an accidental misread of a laboratory value as being within the reference
range when it actually is sufficiently abnormal to preclude study participation by
the subject.
• A Protocol Violation is an intentional act (from the perspective of the PI and study
staff) in which the protocol is not followed. Examples that are reportable to the
IRB include the PI beginning the study before IRB approval, the PI prescribing or
administering the wrong drug on the study, or the study subject being scheduled to
return for follow-up intervention outside the protocol-dictated window as a convenience
to the PI or the study staff.
The Adverse Event Form should be used as you conduct your research study whenever
you encounter a problem or event that is both unanticipated and indicates that the
research places subjects or others at a great risk of harm (including physical, economic,
or social harm) than was previously known or recognized. Unanticipated is defined
as: unexpected (in terms of nature, severity, or frequency) given:
• The research procedures that are described in the protocol-related documents, such
as the IRB-approved research protocol and informed consent document; and
• The characteristics of the subject population being studied.
The adverse event should be reported to the IRB within 10 business days of the event.